Using national clinical guidelines to reduce practice variation – the case of Denmark
Abstract
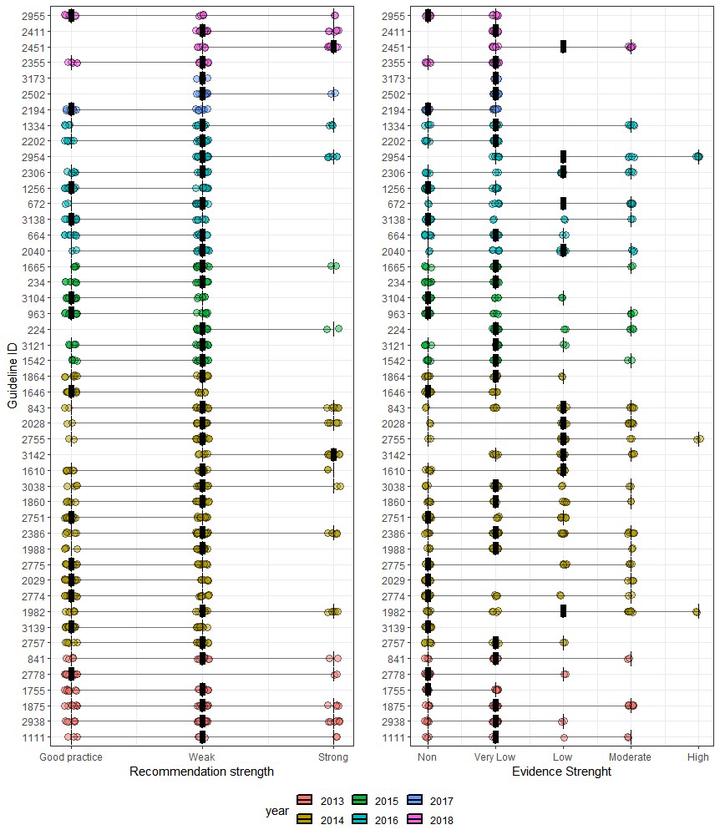
The use and usefulness of clinical practice guidelines to reduce clinical variation remains a contested topic. Between 2012 and 2017, the Danish National Board of Health embarked on an ambitious project to reduce clinical variation with a large-scale investment in developing a set of national clinical guidelines. Forty-seven clinical guidelines where developed during this period using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. In selecting topics for guideline development, the programme prioritised areas where clinical uncertainty existed. Consequently, because little clinical evidence could be identified in these areas, the programme mostly provided weak recommendations for practice. In addition, the initial idea of considering cost effectiveness was abandoned. Finally, no system was put in place to monitor guideline compliance. It is therefore currently unknown whether the investment in developing national clinical guidelines in Denmark will achieve its aim of reducing unwanted practice variations and represents value for money.